What is Nickel Cadmium Battery
Nickel-cadmium batteries are galvanic rechargeable current sources that were invented in 1899 in Sweden by Waldmar Jungner. Before 1932, their practical use was very limited due to the high cost of the metals used compared to lead-acid batteries.
Improvements in their production technology led to a significant improvement in their performance characteristics and made it possible in 1947 to create a sealed, maintenance-free battery with excellent parameters.
Operating principle and design of Ni-Cd battery
These batteries produce electrical energy through the reversible process of interaction of cadmium (Cd) with nickel oxide-hydroxide (NiOOH) and water, which results in the formation of nickel hydroxide Ni(OH)2 and cadmium hydroxide Cd(OH)2, which causes the appearance of electromotive force.
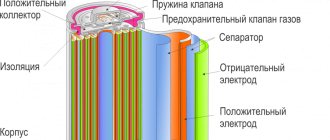
Ni-Cd batteries are produced in sealed cases containing electrodes separated by a neutral separator containing nickel and cadmium in a solution of a jelly-like alkaline electrolyte (usually potassium hydroxide, KOH).
The positive electrode is a steel mesh or foil coated with nickel oxide-hydroxide paste mixed with conductive material
The negative electrode is a steel mesh (foil) with pressed porous cadmium.
One nickel-cadmium cell is capable of producing a voltage of about 1.2 volts, so to increase the voltage and power of batteries, their design uses many parallel-connected electrodes separated by separators.
Technical characteristics and types of Ni-Cd batteries
Ni-Cd batteries have the following technical characteristics:
- the discharge voltage of one element is about 0.9-1 volts;
- the rated voltage of the element is 1.2 v; to obtain voltages of 12v and 24v, a series connection of several elements is used;
- full charge voltage – 1.5-1.8 volts;
- operating temperature: from -50 to +40 degrees;
- number of charge-discharge cycles: from 100 to 1000 (in the most modern batteries - up to 2000), depending on the technology used;
- self-discharge level: from 8 to 30% in the first month after a full charge;
- specific energy intensity – up to 65 W*hour/kg;
- service life is about 10 years.
Ni-Cd batteries are produced in various cases of standard sizes and in non-standard designs, including disk and sealed form.
Parameters [edit | edit code ]
- Theoretical energy content: 237 Wh/kg
- Specific energy intensity: 45–65 Wh/kg
- Specific energy density: 50–150 Wh/dm³
- Specific power: 150…500 W/kg
- EMF = 1.37 V
- Operating voltage = 1.35…1.0 V
- Normal charging current = 0.1…1 C, where C is the capacity
- Service life: about 100-900 charge/discharge cycles.
- Self-discharge: 10% per month
- Operating temperature: −50…+40 °C
Currently, the use of nickel-cadmium batteries is severely limited for environmental reasons, so they are used only where the use of other systems is impossible, namely in devices characterized by high discharge and charging currents. A typical flying model battery can be charged in half an hour and discharged in five minutes. Thanks to the very low internal resistance, the battery does not heat up even when charging with a high current. Only when the battery is fully charged does a noticeable warm-up begin, which is used by most chargers as a signal for the end of charging. Structurally, all nickel-cadmium batteries are equipped with a durable sealed housing that can withstand internal gas pressure under harsh operating conditions.
The discharge cycle starts at 1.35 V and ends at 1.0 V (respectively 100% capacity and 1% remaining capacity)
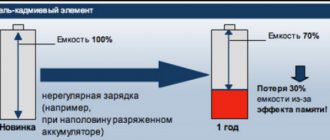
The electrodes of nickel-cadmium batteries are made either by stamping from sheets or by pressing from powder. Pressed electrodes are more technologically advanced, cheaper to produce and have higher operating capacity, which is why all household batteries have pressed electrodes. However, pressed systems are subject to the so-called “memory effect”. The memory effect occurs when the battery is charged before it is actually discharged.
In the electrochemical system of the battery, an “extra” electrical double layer appears and its voltage decreases by 0.1 V. A typical controller of a device using a battery interprets this decrease in voltage as a complete discharge of the battery and reports that the battery is “bad”. There is no real reduction in energy consumption, and a good controller can ensure full use of the battery capacity. However, in a typical case, the controller encourages the user to perform more and more charging cycles. And this leads to the fact that the user, with the best of intentions, “kills” the battery with his own hands. That is, we can say that the battery fails not so much from the “memory effect” of pressed electrodes, but from the “memory effect” of inexpensive controllers.
A household nickel-cadmium battery, discharged and charged with weak currents (for example, in a TV remote control), quickly loses capacity, and the user considers it to be out of order. Likewise, a battery that has been recharged for a long time (for example, in an uninterruptible power supply system) will lose capacity, although its voltage will be correct. That is, you cannot use a nickel-cadmium battery in buffer mode. However, one deep discharge cycle and subsequent charging will fully restore the battery capacity.
Read also: How to connect a 380 to 220 compressor
During storage, NiCd batteries also lose capacity, although they retain the output voltage. To avoid incorrect sorting when removing batteries from storage, it is recommended to store them in a discharged state - then after the first charge the batteries will be completely ready for use. To completely discharge the battery and equalize the voltages on each discharged element, you can connect a chain of two silicon diodes and a resistor to each element, thereby limiting the voltage to 1-1.1 V per element. In this case, the voltage drop across each silicon diode is 0.5–0.7 V, so you must select diodes for the chain manually, using, for example, a multimeter. After long-term storage of the battery, it is necessary to carry out two or three charge/discharge cycles with a current numerically equal to the nominal capacity (1C) so that it enters the operating mode and works at full efficiency.
Where are nickel cadmium batteries used?
These batteries are used in devices that consume high current and also experience high loads during operation in the following cases:
- on trolleybuses and trams;
- on electric cars;
- on sea and river transport;
- in helicopters and airplanes;
- in power tools (screwdrivers, drills, electric screwdrivers and others);
- electric shavers;
- in military equipment;
- portable radios;
- in radio-controlled toys;
- in diving lights.
Currently, due to stricter environmental requirements, most batteries of popular sizes (AAA and others) are produced using nickel-metal hydride and lithium-ion technologies. At the same time, there are still many NiCd batteries of various sizes in use that were released several years ago.
Ni-Cd cells have a long service life, which sometimes exceeds 10 years, and therefore this type of battery can still be found in many electronic devices, in addition to those listed above.
Pros and cons of Ni-Cd battery
This type of battery has the following positive characteristics:
- long service life and number of charge-discharge cycles;
- long service life and storage;
- fast charging capability;
- ability to withstand heavy loads and low temperatures;
- maintaining performance in the most unfavorable operating conditions;
- low cost;
- the ability to store these batteries in a discharged state for up to 5 years;
- average overcharge resistance.
At the same time, nickel-cadmium power supplies have a number of disadvantages:
- the presence of a memory effect, manifested in loss of capacity when charging the battery without waiting for complete discharge;
- the need for preventive maintenance (several charge-discharge cycles) to reach the full capacity;
- complete restoration of the battery after long-term storage requires three to four full charge-discharge cycles;
- high self-discharge (about 10% in the first month of storage), leading to almost complete discharge of the battery within a year of storage;
- low energy density compared to other batteries;
- the high toxicity of cadmium, due to which they are banned in a number of countries, including the EU, the need to dispose of such batteries using special equipment;
- greater weight compared to modern batteries.
How to properly discharge the battery
Regardless of whether slow or fast charging is used, care must be taken to ensure that none of the NiCd cells are overcharged. Therefore, it is necessary to be able to determine the end of the charge. There are several methods to achieve this.
- Basic charger: Some basic NiCd chargers that can be purchased simply charge at around C/10. They do not include a timer and assume the user removes the charge when the cell is charging. This mode is not entirely satisfactory, since the cells will be overloaded if the user forgets and become corrupted as a result. There is also no way to know the exact charging status before starting charging.
- Elapsed Time/Timer: Some of the most basic chargers assume that the cells will need a full charge, and by knowing their capacity, they can be given a charge within a given time. This is a simple way to charge nickel-cadmium cells and batteries. One of the main disadvantages of this form of charging termination is that it assumes that all batteries are completely discharged before charging them. To ensure batteries are discharged, the charger may place the cell in a discharge cycle. This is not a particularly accurate method of recharging batteries and cells because the amount of charge they can hold varies over their useful life. However, this is better than not having any form of charge termination.
- Voltage Signature: Voltage Signature NiCd chargers use the voltage signature of the NiCd cell to determine where it is within its charge cycle. It is found that when a NiCd battery is fully charged, there is a slight voltage drop across the terminals. Microprocessor chargers are able to monitor the voltage and determine the full charge point when they stop the charging process. This form of NiCd charge termination is often called negative delta voltage, NDV. It provides the best performance when using fast charging because the negative delta voltage point is more obvious when using fast charging.
- Temperature increase. The method for determining the end time of fast charging is a temperature measurement method. The problem is that this is inaccurate because the core of the cell will be at a much higher temperature than the periphery. For normal charging rates, the rate of temperature rise may not be sufficient to accurately determine.
Difference between Ni-Cd and Li-Ion or Ni-Mh sources
Batteries with active components including nickel and cadmium have a number of differences from more modern lithium-ion and nickel-metal hydride power sources:
- Ni-Cd elements, unlike Li-Ion and Ni-Mh options, have a memory effect and have a lower specific capacity with the same dimensions;
- NiCd sources are more unpretentious, remain operational at very low temperatures, and are many times more resistant to overcharging and strong discharge;
- Li-Ion and Ni-Mh batteries are more expensive, they are afraid of overcharging and strong discharge, but they have less self-discharge;
- the service life and storage life of Li-Ion batteries (2-3 years) is several times less than that of Ni-Cd products (8-10 years);
- Nickel-cadmium sources quickly lose capacity when used in buffer mode (for example, in a UPS). Although they can then be fully restored by deep discharging and charging, it is better not to use Ni Cd products in devices where they are constantly recharged;
- The identical charging mode of Ni-Cd and Ni-Mh batteries allows you to use the same chargers, but you need to take into account the fact that nickel-cadmium batteries have a more pronounced memory effect.
Based on the existing differences, it is impossible to make an unambiguous conclusion about which batteries are better, since all elements have both strengths and weaknesses.
New battery in old case.
If you were unable to restore the old battery, you can make a battery for a screwdriver at home. For this purpose, you will have to pick up a new battery (or set of cells); there is an option of a higher class that is not a charger for it. Only for nickel-cadmium (nicd) batteries! For about six months, the batteries in my screwdriver failed, no matter how long they sat on charge. The main thing is to keep within the dimensions of the battery case.
If the total battery rating of the new charger is the same, it is enough to make an adapter using the old charger, especially since you will not need it anyway.
If you have a charger for individual cells, you will have to remove them from the case to charge them. There are no more extreme methods - nettle, attaching a battery to a screwdriver that does not fit the form factor.
Unfortunately, this is rather a temporary solution; it’s better to use the old case without choosing new batteries of the appropriate size.
Some people use the “garage option” - using an old starter battery from a car. This popular rumor method is quite applicable, alas, there are some limitations. Some screwdrivers have an operating motor voltage of eighteen volts. How does a battery for a screwdriver work? Is it possible to solder batteries using regular batteries? This corresponds to a fifteen-can battery.
A fully charged car battery produces twelve volts; the screwdriver will not work. If your electrical appliance has a voltage of 14.5 volts - that is, the battery consists of twelve elements, the engine will work. Here is your solution to the problem.
As we now understand, a broken screwdriver battery is not yet a reason to buy a new battery. Everything for converting screwdriver batteries to lithium in Ufa, original high-current 18650 batteries, bms protection boards, pcm, pcb protection boards, balancing boards, control boards. If you have the patience and basic skills in electrical engineering, you can repair the battery at home. In other words, entrust this procedure to a service center. It will turn out to be somewhat more expensive - alas, it will still be cheaper than buying a new battery.
Skip the talk about the benefits of screwdrivers, you can go straight to the topic. The battery or accumulator is the biggest concern for screwdriver owners. It is better to handle them strictly according to the instructions. In the event of a breakdown, there is only one solution - buy a new battery. But you can temporarily try to use the problem battery, for which a number of tips are offered here. We emphasize that repairing the battery is a temporary solution, but first you need to accurately investigate the causes of the malfunction.
Operating rules
During operation, a number of changes occur in Ni Cd power supplies, which lead to a gradual deterioration in performance and, ultimately, to loss of performance:
- the useful area and mass of the electrodes decreases;
- the composition and volume of the electrolyte changes;
- the separator and organic impurities decompose;
- water and oxygen are lost;
- Current leaks appear due to the growth of cadmium dendrites on the plates.
In order to minimize damage to the battery that occurs during its operation and storage, it is necessary to avoid adverse effects on the battery that are associated with the following factors:
- charging an incompletely charged battery leads to a reversible loss of its capacity due to a decrease in the total area of the active substance as a result of crystal formation;
- regular strong overcharging, which leads to overheating, increased gas formation, loss of water in the electrolyte and destroys the electrodes (especially the anode) and the separator;
- undercharging leading to premature battery depletion;
- long-term operation at very low temperatures leads to a change in the composition and volume of the electrolyte, the internal resistance of the battery increases and its performance characteristics deteriorate, in particular the capacity decreases.
With a strong increase in pressure inside the battery as a result of rapid charging with a high current and severe degradation of the cadmium cathode, excess hydrogen can be released into the battery, which leads to a sharp increase in pressure that can deform the case, disrupt the assembly density, increase internal resistance and reduce operating voltage.
In batteries equipped with an emergency pressure relief valve, the risk of deformation can be prevented, but irreversible changes in the chemical composition of the battery cannot be avoided.
Ni Cd batteries must be charged with a current of 10% (if fast charging in special batteries is necessary - with a current of up to 100% in 1 hour) of their capacity (for example, 100 mA with a capacity of 1000 mAh) for 14-16 hours. The best mode for discharging them is with a current equal to 20% of the battery capacity.
Features of use
Nickel-cadmium coulometric charging efficiency is about 83% for fast charging (C/1 to C/0.24) and 63% for C/5 charging. This means that at C/1 you should be using 120 amp-hours at every 100 amp hours you receive. The slower you charge, the worse it gets. In C/10 it is 55%, in C/20 he can get less than 50%. (These numbers are just to give you an idea, battery manufacturers vary).
When the charge is complete, oxygen begins to be generated at the nickel electrode. This oxygen diffuses through the separator and reacts with the cadmium electrode to form cadmium hydroxide. This causes a drop in cell voltage, which can be used to determine the end of the charge. This so-called minus delta V/delta t kick, which indicates the end of the charge, is much less pronounced in NiMH than NiCad and is very temperature dependent. Many of the chargers listed here use a complex algorithm that uses -deltaV to accurately charge NiMH and NiCad packs.
How to restore Ni Cd battery
Nickel-cadmium power supplies in case of loss of capacity can be almost completely restored using a complete discharge (up to 1 volt per element) and subsequent charging in standard mode. This battery training can be repeated several times to fully restore their capacity.
If it is impossible to restore the battery by discharging and charging, you can try to restore it using short current pulses (tens of times the capacity of the element being restored) for several seconds. This effect eliminates the internal short circuit in the battery cells that occurs due to the growth of dendrites by burning them out with a strong current. There are special industrial activators that carry out such an effect.
Complete restoration of the original capacity of such batteries is impossible due to irreversible changes in the composition and properties of the electrolyte, as well as degradation of the plates, but it makes it possible to extend the service life.
The recovery method at home consists of the following steps:
- a wire with a cross-section of at least 1.5 square millimeters connects the minus of the element being restored to the cathode of a powerful battery, for example a car battery or one from UPS;
- a second wire is securely attached to the anode (plus) of one of the batteries;
- for 3-4 seconds, the free end of the second wire is quickly touched to the free positive terminal (with a frequency of 2-3 touches per second). In this case, it is necessary to prevent welding of the wires at the connection point;
- a voltmeter is used to check the voltage on the source being restored; if it is absent, another restoration cycle is performed;;
- when an electromotive force appears on the battery, it is charged;
In addition, you can try to destroy the dendrites in the battery by freezing them for 2-3 hours and then sharply tapping them. When frozen, dendrites become brittle and are destroyed by impact, which could theoretically help get rid of them.
There are also more extreme restoration methods that involve adding distilled water to old elements by drilling out their housing. But fully ensuring the tightness of such elements in the future is very problematic. Therefore, you should not save money and expose your health to the risk of poisoning with cadmium compounds due to the gain of several cycles of operation.
Completion of sterilization
After the required time has passed, gradually reduce the pressure, gradually reducing the heat until the heat source is completely turned off. Allow the unit to cool to a temperature of no more than 30°C, then slowly release the pressure using the nipple. Do not allow sudden heating and cooling, sudden release or increase in pressure - the jars may burst.
Before opening the lid, check the pressure release valve once to make sure that the pressure in the autoclave and outside are equalized. If nothing happens, you can safely open the lid.
Open the lid and remove the jars. One laying and bringing the canned food to cooking takes 3-3.5 hours. As a rule, experienced people do this in the afternoon and by the evening they turn off the autoclave and then leave it to cool in this position until the morning.
When the sun rises, you can take out the finished jars, which will then be a wonderful delicacy for your table!
After you have studied the instructions for using the autoclave, you can begin preparing dishes, including: fish and meat stews, vegetable preparations, homemade pickles, jams and preserves.
Storage and disposal
It is better to store nickel-cadmium batteries in a discharged state at a low temperature in a dry place. The lower the storage temperature of such batteries, the lower their self-discharge. High-quality models can be stored for up to 5 years without significant damage to technical characteristics. To put them into operation, it is enough to charge them.
The harmful substances contained in one AA battery can pollute about 20 square meters of territory. To safely dispose of NiCd batteries, they must be taken to recycling points, from where they are transported to factories, where they must be destroyed in special sealed ovens equipped with filters that trap toxic substances.
History of creation
Full alkaline batteries were developed in the late 19th century by Swiss scientist Waldemar Junger. In this case, nickel was assigned the role of a positive electrode, and cadmium - a negative one.
A couple of years later, the design of the first Ni-Cd battery was improved by Edison. He suggested making the negative electrode out of iron to reduce the cost of the battery. However, even after this, Ni-Cd batteries remained so expensive that their commercial production was delayed for 50 years.
In 1932, scientists Schlecht and Ackerman proposed pressed anode technology, which could significantly increase the battery current and its durability . These Ni-Cd batteries went into production only after Newman developed a sealed case for them. This happened in 1947.
Read also: Measuring luminous flux in lumens