How to charge Ni-Cd batteries - the purpose of the batteries
NiCad and NiMH batteries are some of the most difficult batteries to charge. While with lithium ion and lead acid batteries you can control overcharging by simply setting the maximum charge voltage, nickel batteries do not have a "float charge" voltage. Thus, charging is based on the flow of current through the battery. The voltage for this is not set in stone like other batteries.
This makes these cells and batteries particularly difficult to charge in parallel. This is because you cannot be sure that each cell or stack has the same resistance and so some will draw more current than others, even when they are full. This means you need to use a separate charging circuit for each row in the parallel block, or balance the current in some other way, such as using resistors of such a value that it dominates the current control.
Features of use
Nickel-cadmium coulometric charging efficiency is about 83% for fast charging (C/1 to C/0.24) and 63% for C/5 charging. This means that at C/1 you should be using 120 amp-hours at every 100 amp hours you receive. The slower you charge, the worse it gets. In C/10 it is 55%, in C/20 he can get less than 50%. (These numbers are just to give you an idea, battery manufacturers vary).
When the charge is complete, oxygen begins to be generated at the nickel electrode. This oxygen diffuses through the separator and reacts with the cadmium electrode to form cadmium hydroxide. This causes a drop in cell voltage, which can be used to determine the end of the charge. This so-called minus delta V/delta t kick, which indicates the end of the charge, is much less pronounced in NiMH than NiCad and is very temperature dependent. Many of the chargers listed here use a complex algorithm that uses -deltaV to accurately charge NiMH and NiCad packs.
Special and universal chargers
Many users are interested in the question of how to charge the battery of a Ni-Cd screwdriver. In this case, a conventional device designed for AA batteries will not work. A special charger is most often supplied with a screwdriver. This is what should be used when servicing the battery. If there is no charger, you should purchase equipment for batteries of the presented type. In this case, only the screwdriver battery can be charged. If you use batteries of different types, it is worth purchasing universal equipment. It will allow you to service autonomous energy sources for almost all devices (cameras, screwdrivers, and even batteries). For example, it will be able to charge Ni-Cd batteries iMAX B6. This is a simple and useful device in the household.
Nickel cadmium batteries operating and charging instructions
Nickel-cadmium battery manufacturers do not fully format their batteries before shipping to prevent them from deteriorating during storage. As a result, it is best to give new batteries a slow charge before use. This usually takes from 15 to 24 hours. This ensures that each cell has the same charge level as it self-discharges at different rates during transport.
In addition, it was found that the performance of new cells reaches its optimal value only after a series of charge/discharge cycles. Typically, cells should reach their specified performance levels after five to ten discharge cycles.
Additionally, peak capacity may be reached after approximately 100 or more charge/discharge cycles, after which performance will begin to decline.
This assumes that nickel-cadmium batteries are charged and discharged as required and are not subject to abuse.
Storage Features
Operating instructions for cadmium batteries have been prepared by specialists. The instructions describe how to store power supplies. Several basic rules have been highlighted.
Ni cd sources can only be stored when completely discharged. For these purposes, chargers that are equipped with the appropriate function are used. For emptying, incandescent lamps with the appropriate number of amperes are also used.
Batteries that are properly prepared can be stored for a long time. Temperature changes do not affect the condition and performance.
Premises are used to store nickel-cadmium batteries. After all, temperature fluctuations do not provoke discharge or the launch of irreversible processes.
Although nickel-cadmium batteries are stored for a long time, at a certain stage there is a need for disposal. To do this, you should contact an organization that performs similar processes.
The efficiency of nickel-cadmium batteries is difficult to overestimate. They are equipped with portable tools used in everyday life and in industry. With proper handling, compliance with safety precautions and operating conditions, the period of use exceeds five years.
How to properly discharge the battery
Regardless of whether slow or fast charging is used, care must be taken to ensure that none of the NiCd cells are overcharged. Therefore, it is necessary to be able to determine the end of the charge. There are several methods to achieve this.
- Basic charger: Some basic NiCd chargers that can be purchased simply charge at around C/10. They do not include a timer and assume the user removes the charge when the cell is charging. This mode is not entirely satisfactory, since the cells will be overloaded if the user forgets and become corrupted as a result. There is also no way to know the exact charging status before starting charging.
- Elapsed Time/Timer: Some of the most basic chargers assume that the cells will need a full charge, and by knowing their capacity, they can be given a charge within a given time. This is a simple way to charge nickel-cadmium cells and batteries. One of the main disadvantages of this form of charging termination is that it assumes that all batteries are completely discharged before charging them. To ensure batteries are discharged, the charger may place the cell in a discharge cycle. This is not a particularly accurate method of recharging batteries and cells because the amount of charge they can hold varies over their useful life. However, this is better than not having any form of charge termination.
- Voltage Signature: Voltage Signature NiCd chargers use the voltage signature of the NiCd cell to determine where it is within its charge cycle. It is found that when a NiCd battery is fully charged, there is a slight voltage drop across the terminals. Microprocessor chargers are able to monitor the voltage and determine the full charge point when they stop the charging process. This form of NiCd charge termination is often called negative delta voltage, NDV. It provides the best performance when using fast charging because the negative delta voltage point is more obvious when using fast charging.
- Temperature increase. The method for determining the end time of fast charging is a temperature measurement method. The problem is that this is inaccurate because the core of the cell will be at a much higher temperature than the periphery. For normal charging rates, the rate of temperature rise may not be sufficient to accurately determine.
The process of discharging and charging Ni-Cd batteries
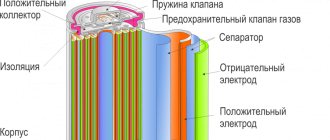
During the battery charging process, a chemical reaction occurs on the positive electrode, made of nickel oxide, releasing a free electron. Additional reactions take place at the cadmium negative electrode.
When the cell is recharged, oxygen atoms are released, which are then fed through a porous separator to the negative pole for subsequent reduction. The constancy of the recovery cycle ensures that the gas pressure inside the closed housing is maintained stable.
During overdischarge, hydrogen atoms are formed on the negative electrode, which is then oxidized on the nickel positive element. Due to the low speed of this process, gas accumulation is possible. To eliminate the effect of hydrogen evolution in N-Cd batteries, negative electrodes are always used, which have a larger volume than positive electrodes.
Nickel-cadmium battery discharge process
The discharge procedure for batteries based on a nickel-cadmium composition is influenced by several factors:
- configuration and structure of electrodes;
- separator layout and thickness;
- the amount of electrolyte and its chemical composition;
- assembly density;
- design features of the battery.
The housing configuration and electrode area are taken into account when choosing the type of battery that suits the operating conditions. For example, disk batteries with an increased cross-section of electrodes made using pressing technology are used under conditions of prolonged discharge. The devices provide a smooth decrease in capacity and voltage to 1.1 V. The residual capacity is up to 10%, it drops during further discharge to 1 V.
The design of the cylindrical element does not allow increasing the discharge current to values above 20% of the rated capacity.
Brand of Ni-Cd batteries.
The reason is the impossibility of ensuring uniform functioning of the active mass over the entire cross-section of the electrodes.
To eliminate the shortcoming, it is practiced to reduce the diameter of the electrodes while simultaneously increasing the number of parts. When using 4 elements, the current increases to 55-60% of the battery capacity.
To increase operating efficiency, nickel-cadmium batteries with electrodes made of metal-ceramic composite are used. The parts are characterized by reduced internal resistance, ensuring that the voltage is maintained at least 1.2 V until the discharge reaches 90% of the capacity declared by the manufacturer.
When the terminal voltage decreases to 1.0 V, the battery capacity is reduced to 3% of the starting value. When connecting an external load, the discharge current exceeds the rated capacity of the battery cells by 3-5 times.
Cylindrical AA or AAA batteries are equipped with electrodes of a roll design. The devices provide current in the circuit up to 10 times the rated capacity. To ensure maximum performance, it is necessary to maintain the temperature of the current source in the range of 18-22°C.
When heated, the capacity of the elements decreases slightly; when the battery is cooled to negative temperatures, the capacity begins to decrease (proportional to the current). This effect occurs due to an increase in the resistance of the electrolyte and the electrode material.
With a further decrease in temperature, crystals begin to form in a closed volume of the electrolyte. The composition and amount of solid fractions depend on the state of the element and the degree of cooling. When the electrolyte completely freezes, electrochemical processes stop, which leads to a drop in voltage to zero.
Manufacturers of nickel-cadmium batteries do not recommend using the products at temperatures below -20°C. There are modifications designed for cooling down to -40°C, but how long the battery will last under such conditions is unknown.
Charging process for nickel-cadmium batteries
When the capacity of nickel-cadmium current sources is restored, the degree of charging is forced to be limited. During the charging process, oxygen is released, which increases the pressure inside the battery case; the electrochemical processes that take place reduce the efficiency of using the incoming current.
Part of the supplied electricity is converted into heat; the battery design includes a drain valve that releases excess gas when the pressure rises above the permissible level.
The longevity of the battery depends on the current used to charge it. To ensure maximum effect, the current strength is set at 1.6-2.0 of the rated capacity of the charged element. The design of the battery allows charging at temperatures from 0° to 40°C, but it is recommended to perform the operation when heated to 10-30°C.
When trying to charge a frozen battery, the resulting oxygen is not absorbed by the negative electrode material, which leads to an increase in pressure and deformation of the metal casing of the battery.
9.6V 1400mAh Ni-Cd battery
As the temperature rises, the release of oxygen ions on the positive electrode occurs faster, which speeds up the procedure for replenishing the capacity. While maintaining a stable temperature, the charging intensity is controlled by the current supplied to the terminals, which changes the intensity of ion release.
In this case, the absorption rate does not depend on the degree of heating; this parameter is determined by the design of the nickel-cadmium element.
Since the intensity of oxygen absorption depends on the configuration of the electrodes, the design of the separator and the volume of electrolyte, it is possible to create batteries that allow accelerated charging. For this purpose, current sources with an increased number of electrodes having a reduced cross-section are used. For example, cylindrical cells charge 2-3 times faster than flat batteries.
There are also methods for charging nickel-cadmium batteries with degraded electrolyte. A hole is drilled in the element body through which distilled water is pumped. If a battery bank assembled from several batteries is being restored, then parts with a voltage at the terminals of about 0 V are first determined.
Batteries filled with water are kept at room temperature for 10-12 hours, then voltage is applied to the terminals, allowing electrochemical processes to be activated.
After a voltage other than 0 V appears at the outputs, standard charging is performed. It is recommended to keep the current sources for 2-3 days, and then carry out a control voltage measurement. If it falls, distilled water is refilled (the volume depends on the size of the case).
If the voltage has not decreased, the holes are sealed, and the elements are charged and discharged 2-3 times; if necessary, the components are assembled into a single jar.
To what level should it be discharged?
When the battery reaches the end of its charge, oxygen begins to form at the electrodes and recombine at the catalyst. This new chemical reaction creates heat, which can be easily measured using a thermistor. This is the safest way to determine the end of charge during fast charging. This method is often used with multi-cell batteries, and 20, 30 and 40 battery chargers use a thermistor here.
Nickel-cadmium battery chargers must shut off charge when the temperature exceeds the maximum charging temperature, typically 45 degrees C for controlled fast charging and 50 degrees C for fast or overnight fast charging.
How long does it take to charge the battery?
Cylindrical Ni-Cd batteries require 7-8 hours of charging with direct current at a force of 0.2 C. To quickly replenish the capacity, we allow an increase in power to 0.3 C while monitoring the condition of the battery. In this case, the battery will be charged within 3-4 hours. Also, with accelerated capacity replenishment, the recharge must be up to 150% of the initial resource, otherwise the Ni-Cd battery will gain a volume below the required one. In fast charging mode, you should use automatic devices that have temperature and voltage control systems. They protect the battery from overheating and rapid pressure build-up inside the case.
Recommendations for operating batteries of different types.
If the operating and charging rules are followed, nickel-cadmium batteries can last up to 10 years. Depending on the charger, their capacity is replenished in 7-8 hours when DC current is supplied. If Ni-Cd batteries are not fully charged, they are subject to a strong memory effect, so the process must always be completed.
How often should you discharge?
Unlike lead-acid cells, NiCads are charged using a constant current source. Their internal resistance is such that if constant voltage were used, they would draw excessively high currents which could damage the cells.
Typically cells charge at a rate of about C/10. In other words, if their capacity is 1 amp hour, they will charge at a rate of 100 mA. Charging times typically exceed ten hours because not all the energy entering the cell is converted into stored electrical energy.
It is found that during the first stage of charging, up to approximately 70% full charge, the charging process is almost 100% efficient. After that he falls.
Difference between Ni-Cd and Li-Ion or Ni-Mh sources
Batteries with active components including nickel and cadmium have a number of differences from more modern lithium-ion and nickel-metal hydride power sources:
- Ni-Cd elements, unlike Li-Ion and Ni-Mh options, have a memory effect and have a lower specific capacity with the same dimensions;
- NiCd sources are more unpretentious, remain operational at very low temperatures, and are many times more resistant to overcharging and strong discharge;
- Li-Ion and Ni-Mh batteries are more expensive, they are afraid of overcharging and strong discharge, but they have less self-discharge;
- the service life and storage life of Li-Ion batteries (2-3 years) is several times less than that of Ni-Cd products (8-10 years);
- Nickel-cadmium sources quickly lose capacity when used in buffer mode (for example, in a UPS). Although they can then be fully restored by deep discharging and charging, it is better not to use Ni Cd products in devices where they are constantly recharged;
- The identical charging mode of Ni-Cd and Ni-Mh batteries allows you to use the same chargers, but you need to take into account the fact that nickel-cadmium batteries have a more pronounced memory effect.
Based on the existing differences, it is impossible to make an unambiguous conclusion about which batteries are better, since all elements have both strengths and weaknesses.
How to charge nickel-cadmium batteries
Sometimes equipment using nickel-cadmium cells requires the use of fast charging methods.
Typically, charging occurs at a rate of around C. However, you must ensure that NiCd charging is working correctly and that charging stops immediately after charging is complete.
Since the charging efficiency is nearly 100% up to about 70% full charge, the full charging rate is maintained up to this point, after which the charging rate decreases as the temperature rises as the charging efficiency decreases.
Important! It has been found that fast charging for NiCd cells also improves charging efficiency. At a 1C charging rate, the overall charging efficiency of a standard NiCd is around 90%, with a charging time of just over an hour.
Charging conditions for new batteries
Ni cd batteries how to charge?
Advantages
To understand how to properly charge Ni-Cd batteries for a screwdriver, camera, camera and other portable devices, you need to familiarize yourself with the technology of this process. It is simple and does not require special knowledge and skills from the user. Even after storing the battery for a long time, it can be quickly recharged. This is one of the advantages of the presented devices that makes them popular.
Nickel-cadmium batteries have a large number of charge and discharge cycles. Depending on the manufacturer and operating conditions, this figure can reach more than 1 thousand cycles. The advantage of the Ni-Cd battery is its endurance and ability to work under heavy-duty conditions. Even when operating in cold weather, the equipment will work properly. Its capacity does not change under such conditions. At any charge level, the battery can be stored for a long time. Its important advantage is its low cost.
Technical specifications
- resistance R5 within hundreds of ohms;
- stabilization voltage 4.9V;
- voltage stability when the load changes from 20 mA to 1 A;
- upper current limit 0.5 A;
- heating of the transistor no more than 50-60oC;
- charging current 200 mA.
Varieties by charging type
There are two types:
- Automatic. Such devices choose the current and duration themselves; you just need to specify the battery parameters.
- Reversible pulse. Everything is configured manually, which can sometimes speed up the process and sometimes slow it down greatly.
For beginners and those who do not practice professionally, automatic options are recommended.
How to use devices, instructions
Standard algorithm:
- Measure the battery charge with a multimeter.
- If it is below 20%, you can start charging.
- Place the battery in the charging position.
- Select mode, duration, current, resistance.
- Wait until charging finishes.
- Test by measuring with a multimeter.
Some batteries can be charged without harm even if the charge is above 20%.
Stop charging
When learning how to charge Ni-Cd batteries, you need to consider the completion of the process. After the current stops flowing to the electrodes, the pressure inside the battery still continues to increase. This process occurs due to the oxidation of hydroxyl ions at the electrodes.
Over some time, there is a gradual equalization of the rate of oxygen release and absorption at both electrodes. This leads to a gradual decrease in pressure inside the battery. If the recharge was significant, this process will be performed more slowly.